While hypochlorous acid (HOCl) and bleach (sodium hyochlorite, or NaOCl) are both types of chlorine, they are vastly different.
Composition
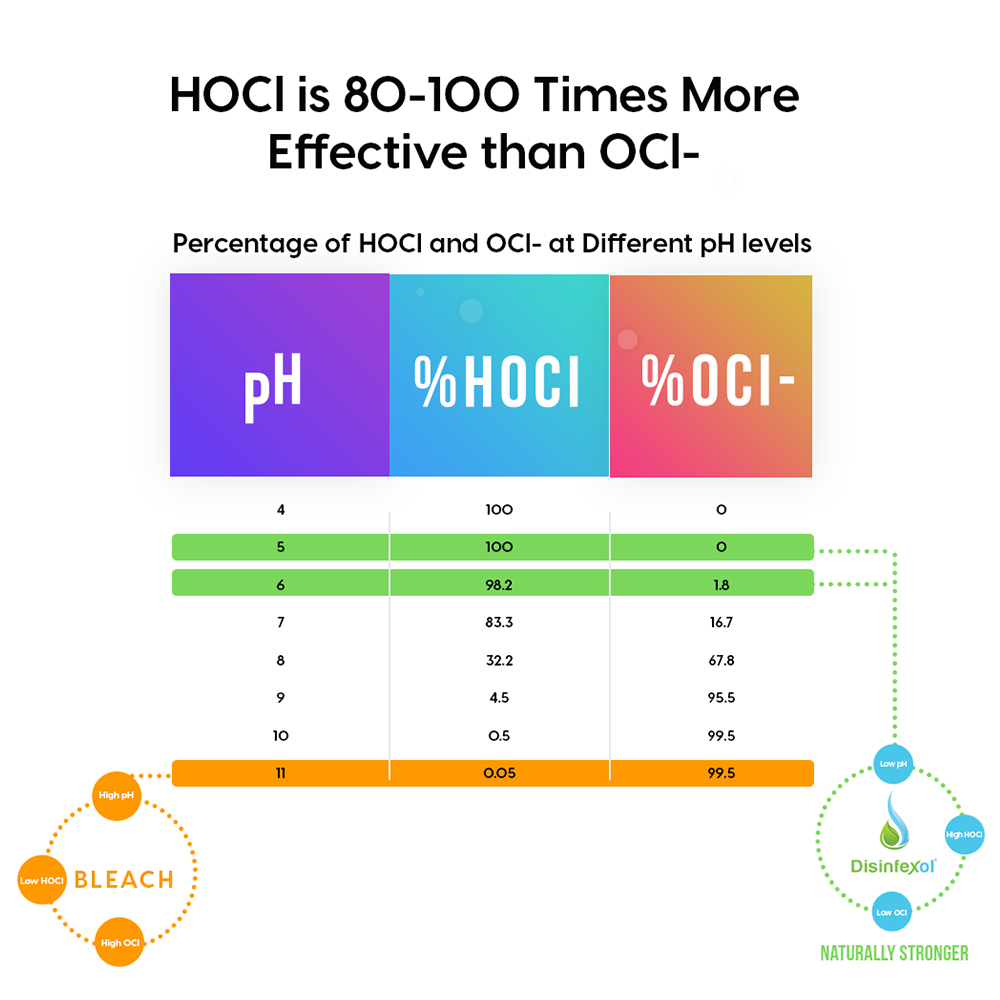
Chlorine in water splits into two forms, hypochlorous acid (HOCl) and Hypochlorite (OCl-). At the high pH, which is the chlorine solution provided by bleach, chlorine is in the hypochlorite OCl- form. As pH rises, less hypochlorous acid and more hypochlorite is in the solution, which means less germ killing power is available.
According to a University of Illinois study, HOCl is 100 times more effective as a sanitizer than the -OCl ion. Even at dilutions as low as 1 ounce of bleach to a gallon of water, the pH of the solution is 10.25 and all of chlorine is in the hypochlorite form (OCl-) ion. HOCl has a different chemical composition than bleach. It is produced in the 5 to 6 pH range and is the molecule that kills microbes.
HOCl is actually a wonder of nature, both inside and outside of the human body. It is a molecule composed of only three natural elements straight from the Periodic Table of Elements – Hydrogen (H), Oxygen (O) and Chlorine (Cl).
It is produced naturally in the white blood cells of all mammals and is transported to the site of injury or infection to aid in the destruction of invading pathogens, making it a biocompatible and eco-friendly disinfectant that is completely safe for humans and animals.
Effectiveness
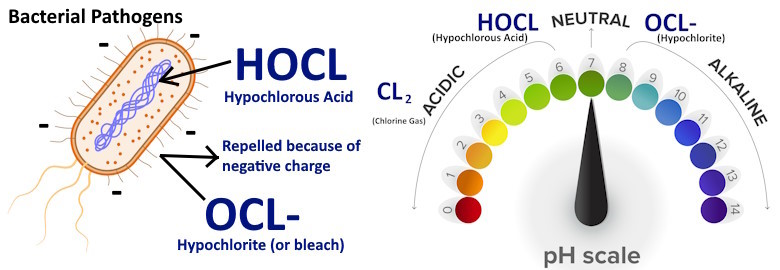
Because HOCl has a neutral charge, it doesn’t repel pathogens. Instead, the negatively charged germs are attracted to it and they drop their defenses. This allows HOCl to easily penetrate the cell walls of viruses, bacteria, and other pathogens, and to kill (inactivate) them from the inside.
Bleach-based products (as well as alcohol and quats), lack a neutral charge. Nearly all harmful microorganisms are negatively charged. Therefore, these lower-performing disinfectants have the disadvantage of repelling dangerous pathogens. Because of this, these other disinfectants are unable to penetrate the cell walls of viruses and germs, making them far less effective at killing these microorganisms than HOCl, which has the advantage of a neutral charge.
Safety
Bleach can be highly corrosive and toxic, causing irritation to skin, eyes and respiratory systems. It requires careful handling and proper ventilation due to its potential to release harmful fumes.
HOCl, on the other hand, is non-toxic and non-irritating. It achieves the lowest level (Category IV) on the EPA’s acute toxicity scale. HOCl is used in organic food production facilities, is safe for humans, animals, plants and waterways (being used to treat drinking water as a much safer alternative to bleach) and is 100% biodegradable.







